Cart (0 Items)
Your cart is currently empty.
View ProductsAre long timelines, inconsistent results, and low yields hindering your monoclonal antibody development? At ProteoGenix, we streamline your path to success with our cutting-edge Verified In-Situ Plate Seeding (VIPS™) technology, reducing development time by more than 50% and delivering clonality reports essential for successful IND submission. Our IP-free, royalty-free cell lines are engineered for stability and high productivity, offering yields exceeding 7g/L. With over 30 years of expertise and 100+ stable cell lines generated, we provide a platform focused on developability. Elevate your research and production with ProteoGenix’s proven solutions.
Advanced VIPS™ Technology
Accelerate custom cell line projects by over 50% with real-time, high-resolution imaging confirming monoclonality using state-of-the-art VIPS™ technology.
Yeilds Guarandeed
Plan for the long-term and secure your investments by choosing a stable cell line development service with yields exceeding 7g/L.
Diverse IP-Free Cell Lines
Benefit from our royalty-free cell lines including Proprietary CHO-1, CHO-S, CHO-DG44, HEK293 or even your preferred cell line… Get a service adapted to your requirements!
Emphasis on developability
Minimize production risks thanks to our extensive early testing of antibody candidates.
Ease of transference
Fast cell line and protocol transference to your CMO partners for cGMP bioproduction.
Protein production experts
500+ monoclonal antibodies developed, 100+ stable cell lines generated, 28+ years of experience in protein production. Choose a leading bioproduction company.
Expression vector construction
Developability Study
Host Cell Transfection and Characterization of Stable Transfected Pools
Best Monoclones Isolation and Selection by VIPS™ Technology
Characterization of the best monoclones
RCB QC and Stability Study
| Step | Content | Timeline | Deliverables |
|---|---|---|---|
| Gene synthesis |
|
1 week |
|
| Transient Expression Evaluation |
|
2 weeks |
|
| Stable pool generation |
|
6 weeks |
|
| Single cell clone screening by VIPS™ |
|
~8-10 weeks |
|
| RCB Preparation and QC |
|
1 week |
|
| RCB Stability Study |
|
~ 4-5 weeks |
|
| Analytics |
|
~ 2-3 weeks |
|
Options:Further evaluation and characterization of the antibody can be done by
The use of Verified In-Situ Plate Seeding (VIPS™) technology in custom cell line development offers several significant technical advantages:
| Cell Lines | Amplification and Selection | Advantages | Applications |
|---|---|---|---|
| Proprietary CHO-K1 | Available with both MTX/DHFR-mediated or Methionine Sulfoximine (MSX)/GS-mediated amplification and selection. | Offers freedom to operate with a one-time fee payment, making it cost-effective for long-term use. | Suitable for the rapid and easy transfer for cGMP production, supporting scalable and flexible biomanufacturing processes. |
| CHO-STM | Utilizes MTX/DHFR-mediated or Methionine Sulfoximine (MSX)/GS-mediated amplification and selection. | No licence fee is required before moving towards commercial use, reducing initial investment costs. | Designed for straightforward scale-up and transfer, facilitating seamless progression from research to commercial production. |
| DG44 | Employs MTX/DHFR-mediated amplification and selection. | No license fees for early-stage use and is renowned for producing FDA-approved biotherapeutics. | Ideal for the bioproduction of therapeutic proteins under stringent regulatory requirements. |
| HEK293 Variants (HEK293, 293F, 293E) | These are human embryonic kidney cells that are versatile for transient and stable expression with high yield. | HEK293 cells are known for rapid growth and high protein yield, making them suitable for both research and commercial scale productions. | Commonly used in the production of viral vectors for gene therapy, vaccines, and recombinant protein products. |
| Customer-Specific Cell Lines | We offer the flexibility to develop and utilize custom cell lines as per client-specific requirements. | Tailored to meet unique project needs, ensuring optimal expression and productivity. | Custom cell lines are particularly useful for specialized therapeutic targets or when proprietary systems are needed for competitive differentiation. |
Antibody genes were optimized for expression in CHO cell lines, synthesized, and subcloned into our proprietary transient expression system.
CHO-K1 cells (30 ml) were transiently transfected, transient pools were grown in 30 ml, and antibody purification was performed using protein G resin.
| Yield: 18.2 mg/L | Quantity produced: 0.18 mg | Purity >90% |
|---|
| A final QC was performed by reduced and non-reduced PAGE analysis confirming the integrity of the antibody |
3 positive stable pools were further characterized in fed-batch experiments and antibodies characterized by SEC-HPLC to confirm purity and detect potential aggregation
| Pool ID | Quantity (mg) | Yield (mg/L) | Purity |
|---|---|---|---|
| 1 | 37.3 | 1243 | >90% |
| 2 | 39.4 | 1313.3 | >90% |
| 3 | 43.6 | 1453.3 | >90% |
| 1L fed-batch production was carried out in 3L flasks and purification was performed using protein A resin |
|---|
| Final yield: 2015.5 mg/L | Final purity: >92% |
Monoclones were isolated using the limiting dilution method in 96-well plates and
expression evaluation was performed by ELISA with anti-Fc antibodies
2 Rounds of limiting dilution
29 Best performing clones
Best monoclones were evaluated by SDS-PAGE and ELISA (Fc-antibody).
ELISA data (below) revealed 10 monoclones with high expression levels.
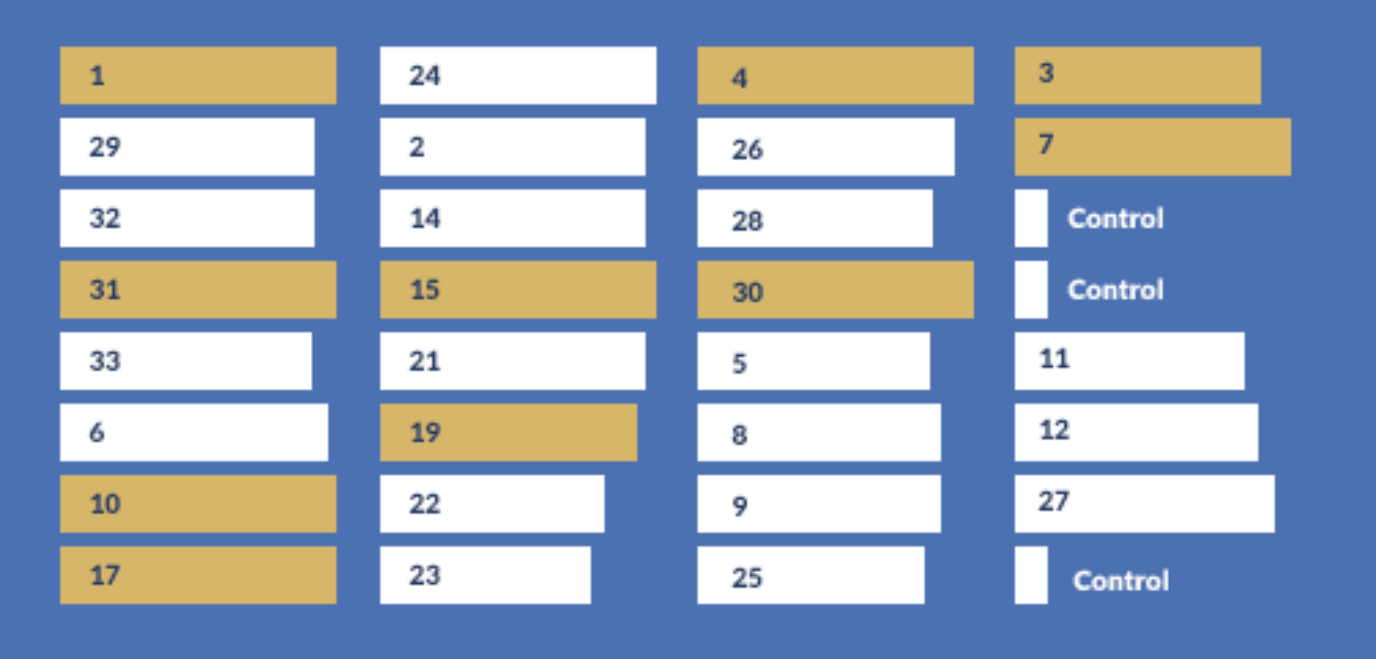
Bar length represents intensity obtained in ELISA, numbers represent the clone ID.
| Clone ID | 1 | 3 | 4 | 7 | 10 | 15 | 17 | 19 | 30 | 31 |
|---|---|---|---|---|---|---|---|---|---|---|
| Yield (g/L) | 2.05 | 2.86 | 1.07 | 1.68 | 4.34 | 2.57 | 3.21 | 2.60 | 1.45 | 1.25 |
Monoclones 3, 10, 17, and 19 maintained good stability and viability in
subsequent tests and were selected for further development
Monoclone 10 was used for initial scale-up with excellent results.
95% of biologics under development fail to reach the market or clinic due to undetected developability issues. Early testing or screening has thus become crucial to overcome low success rates and minimize development risks. Producing monoclonal antibodies in vitro via transient systems is still the best way to produce small amounts of antibodies for measuring:
Detectable problems at this stage can be corrected by additional engineering of antibody leads. These engineering efforts, although laborious, can save considerable costs and time on the long run to license your therapeutic antibodies.
To learn more about stable cell line development, visit our frequently asked questions (FAQs) page. On this page, we cover all seminal principles and knowledge regarding monoclonal antibody production in stable cells and provide detailed insights into ProteoGenix’s unique platform for stable cell line development.
Got a question or need a quote? Message us and we’ll get back to you 48 hours or less.